PRODUCT IDENTIFICATION
H.S. CODE
2930.90
TOXICITY
CLASSIFICATION
PHYSICAL AND CHEMICAL PROPERTIES
MELTING POINT
SOLUBILITY IN WATER
REFRACTIVE INDEX
GENERAL DESCRIPTION & APPLICATIONS
There are some types of rubber accelerators. They are used in combination with each other in accordance with vulcanizing and/or acid-base conditions. Some examples classified by chemical structure are as below;
- Thiazole
- 2-Mercaptobenzothiazole (CAS #: 149-30-4)
- Dibenzothiazole disulfide (CAS #: 120-78-5)
- 2-Mercaptobenzothiazole Zinc salt (CAS #: 155-04-4)
- Sulphenamide
- N-Cyclohexyl-2-benzothiazole sulfenamide (CAS #: 95-33-0)
- N-Oxydienthylene-2-benzothiazole sulfenamide (CAS #: 102-77-2)
- N-tert-butyl-2-benzothiazyl sulfenamide (CAS #: 95-31-8)
- Guanidine
- Diphenyl guanidine (CAS #: 102-06-7)
- Di-o-tolylguanidine (CAS #: 97-39-2)
- Thiuram
- Tetramethyl thiuram disulfide (CAS #: 137-26-8)
- Tetraethyl thiuram disulfide (CAS #: 97-77-8)
- Tetramethyl thiuram monosulfide (CAS #: 97-74-5)
- Isobutyl thiuram disulfide (CAS #: 3064-73-1)
- Tetrabenzylthiuram disulfide (CAS #: 10591-85-2)
- Dipentamethylene thiuramtetrasulfide (CAS #: 120-54-7)
- Dithiocarbamate
- Zinc dimethyl dithiocarbamate (CAS #: 137-30-4)
- Zinc diethyl dithiocarbamate (CAS #: 14324-55-1)
- Zinc dibutyl dithiocarbamate (CAS #: 136-23-2)
- Zinc N-ethyl-dithiocarbamate (CAS #: 14634-93-6)
- Zinc dibenzyl dithiocarbamate (CAS #: 14726-36-4)
- Copper dimethyl dithiocarbamate (CAS #: 137-29-1)
- Thiourea
- Ethylene thiourea (CAS #: 96-45-7)
- N,N'-Diethylthiourea (CAS #: 105-55-5)
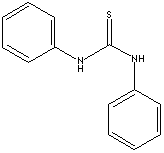
- N-N'-Diphenylthiourea (CAS #: 102-08-9)
Diphenyl Thiourea is used as a primary or secondary vulcanization accelerator. It is used as an intermediate for the synthesis of dye and other organic compounds. It is used as an ore flotation agent.
APPEARANCE
96.0% min
148 C min
ASH
0.3% max
0.3% max
99.9% (150 mesh)
GENERAL DESCRIPTION OF THIOUREA
Thiourea (also called Thiocarbamide or Sulfourea) is the diamide of thiocarbonic acid that resembles urea but contains sulfur instead of oxygen. 'Thio' is a chemical prefix indicates the replacement of an oxygen in an acid radical by sulfur with a negative valence of 2; meaning 'Sulfur' derived from the Greek theion. In fct, thiourea occurs as the mixture of two tautomers: S=C(NH2)2 ( Thiourea) + HS=CNHNH2 (Isothiourea), accordingly, provides three functional groups (mino, imino, and thiol). Thiourea is a lustrous white crystalline compound; estimated melting point is 170-180 C; soluble in water and in polar organic solvents; insoluble in non-polar solvents. The exact melting point and boiling point are not available since rearrangement to ammonium thiocyanate (NH4SCN) occurs at about 135 C and decomposition occurs. It can be prepared by heating ammonium thiocyanate, or by the addition of hydrogen sulfide to cyanamide. The latter is the more common method. Thiourea is used directly in ore filtering, metal refinery and cleaning, isomerization catalyst (conversion of maleic to fumaric acid) and as an additive in fertilizers (to inhibit the nitrification process), drilling auxiliaries, light-sensitive photocopy paper and explosives. It is used as a fixing agent in photography, as a liquefying agent in animal hide glue, as an insecticide, as a textile-treating agent, and as an intermediate to produce other compounds. Thiourea and its derivatives are versatile intermediates for the synthesis of modified thermosetting resins, thiourea dioxide, dyes, flame retardants, vulcanization accelerators, plant protection agents, pesticides, amino resins, peptizing agents, fungicides, hair preparations, dry cleaning chemicals, corrosion inhibitors and thiazole drugs (e.g., antiseptic, thyrotherapeutic, narcotic, and tuberculostatic agents). Dithiobiurea possesses a wide dipole moment and thus is involved in the forming wide metal chelated complexes as the radioactiv-compound which used in radiopharmaceutical imaging, inhibiting enzyme function, kidney function study and to treat toxic metal poisoning. It is used in co-crystals development used in the field of nonlinear optics to generate new coherent wavelengths.